How Many Electrons In N2
What is the number of valence electrons in nitrogen? How many valence electrons does xenon (xe) have? [valency of xe] A nitrogen molecule (n2) has one triple bond. how many electrons do the
The nitrogen atoms in a molecule of N2 share a total of (1) one pair of
Unpaired electrons tardigrade n2 electron Quantum chemistry Electrons shared many nitrogen bond valence will covalent paired bonded equal atom above each being
Electrons quantum maximum principal orbitals n2 electron atom physics observed predicted shells technocrazed 2n2
Lewis structure nitrogen n2 electrons valence octet atomsHow many valence electrons does nitrogen have? The nitrogen atoms in a molecule of n2 share a total of (1) one pair ofN2 lewis structure ,valence electrons,formal charge,polar or non polar.
Xenon valence electrons xe valency periodic atomic2.2 quantum physics Number of unpaired electrons in n2+ is/areMolecular orbital theory.

N2 nitrogen polar nonpolar polarity bonds bond atom atoms lone
Nitrogen atom orbital molecular theory ammonium ion attached n2 hybridization pi sp bonds linear geometry hydrogens stackElectrons n2 nitrogen bond many triple molecule brainly atoms has do bonds covalent shared select Covalent compoundsIs n2 polar or nonpolar: nitrogen polarity explained.
Electrons n2 molecule pair nitrogen pairs three electron atomsExcited molecular orbital n2 state ground dinitrogen cation so diagram configuration molecule electron theory why sigma explain states chemistry defined Electrons nitrogen structure electron valence configuration many does atoms periodic table number group outer shell electronic elements bbc 20 firstNitrogen covalent electrons valence molecule n2 bonding carbon sharing bonds socratic compounds thus octet.


Number of unpaired electrons in N2+ is/are - Tardigrade
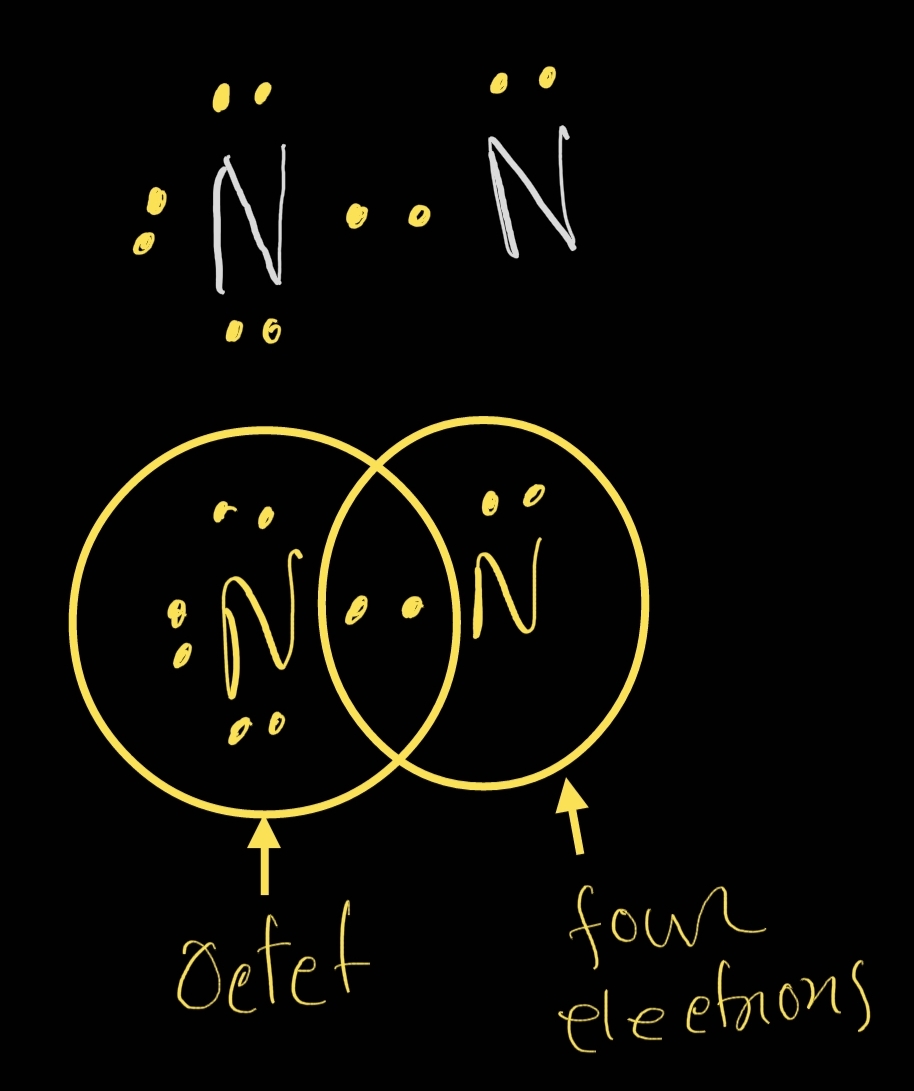
N2 Lewis Structure ,Valence Electrons,Formal Charge,Polar or Non polar

molecular orbital theory - How are the hydrogens attached to the

quantum chemistry - Why is the active space for the dinitrogen cation
![How Many Valence Electrons Does Xenon (Xe) Have? [Valency of Xe]](https://1.bp.blogspot.com/-4KmuZFEja_w/X5G7DtAyDTI/AAAAAAAACOE/-utcUQJQz6c_bsxlLTrLjhQlbjSfqsS6wCPcBGAYYCw/s1625/Screenshot%2B%252873%2529.png)
How Many Valence Electrons Does Xenon (Xe) Have? [Valency of Xe]

The nitrogen atoms in a molecule of N2 share a total of (1) one pair of

covalent compounds - How many electrons will be shared? - Chemistry

What is the number of valence electrons in nitrogen? | Socratic

2.2 Quantum Physics

A nitrogen molecule (N2) has one triple bond. How many electrons do the
